Esketamine Nasal Spray (FDA-Approved Prescription Treatment)
Overview Esketamine nasal spray
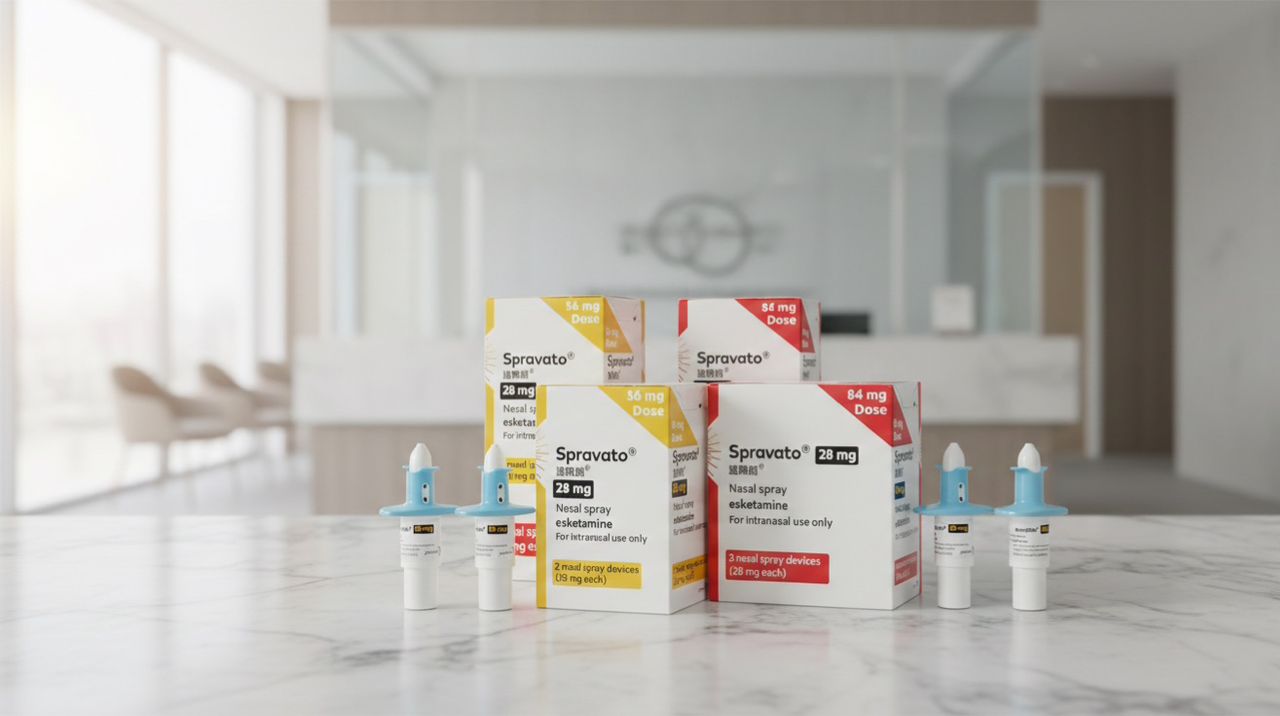
Esketamine nasal spray is an FDA-approved prescription medication used for the treatment of adults with treatment-resistant depression (TRD) and major depressive disorder (MDD) with acute suicidal ideation, when used in conjunction with an oral antidepressant. Marketed under the brand name SPRAVATO®, esketamine represents a novel approach to depression treatment with a unique mechanism of action.
In the United States, esketamine nasal spray is a Schedule III controlled substance and is available only through certified healthcare settings under a strict Risk Evaluation and Mitigation Strategy (REMS) program. Special K Zone provides educational and referral-based access information in full compliance with FDA and DEA regulations.
What Is Esketamine Nasal Spray?
Esketamine nasal spray prescription medication contains esketamine, the S-enantiomer of ketamine. As a NMDA antagonist nasal spray, esketamine differs from traditional antidepressants by targeting glutamate pathways rather than serotonin or norepinephrine systems.
Spravato esketamine nasal spray is administered intranasally under medical supervision and is not dispensed for home use. Patients receive treatment in certified clinics, where they are monitored before, during, and after administration.
How Esketamine Nasal Spray Works
Understanding how esketamine nasal spray works is essential to its clinical role. Esketamine blocks N-methyl-D-aspartate (NMDA) receptors, leading to increased glutamate signaling and enhanced synaptic plasticity in key areas of the brain associated with mood regulation.
This mechanism allows esketamine to produce rapid antidepressant effects, often within hours to days, compared to traditional antidepressants that may take weeks to show benefit. This makes esketamine treatment for depression particularly valuable for patients who have not responded to standard therapies.
FDA Approval and Indications
Patients often ask, “Is esketamine nasal spray FDA approved?”
Yes. Esketamine nasal spray (SPRAVATO®) is FDA-approved for:
-
Treatment-resistant depression (TRD) in adults
-
Major depressive disorder with acute suicidal ideation or behavior (with an oral antidepressant)
Additionally, is esketamine legal in the US?
Yes. Esketamine is legal for medical use when prescribed and administered in accordance with FDA guidelines and the REMS program.
Esketamine Nasal Spray Dosing Protocol
The esketamine nasal spray dosing protocol is determined by a licensed healthcare provider and follows FDA-approved schedules. Treatment typically consists of:
-
Induction phase: Twice-weekly administration for the initial weeks
-
Maintenance phase: Reduced frequency based on patient response
Dosing is based on patient factors and clinical response. Because of potential side effects, patients must remain under observation for a designated period after each treatment session.
Safety Information and Warnings
Patients and providers should carefully review esketamine nasal spray side effects and warnings. Common or clinically observed effects may include:
-
Dissociation or perceptual changes
-
Sedation or drowsiness
-
Increased blood pressure
-
Nausea or dizziness
Due to these effects, esketamine must be administered in a controlled medical setting. Patients are advised not to drive or operate machinery on the day of treatment. Individuals with certain cardiovascular or psychiatric conditions may require additional evaluation.
REMS Program and Regulatory Compliance
Esketamine nasal spray is subject to the esketamine REMS program, which ensures:
-
Administration only in certified healthcare facilities
-
Direct supervision by trained medical professionals
-
Mandatory post-dose monitoring
-
Controlled distribution and recordkeeping
This program is designed to maximize patient safety while allowing access to an innovative FDA-approved treatment.
Accessing Esketamine Treatment in the US
While patients cannot directly purchase esketamine nasal spray for home use, they can access treatment through certified US clinics and providers. Special K Zone offers compliant educational resources and guidance to help patients understand eligibility, referral processes, and what to expect during treatment.
All access pathways comply with FDA, DEA, and state-level regulations.
Frequently Asked Questions
Is esketamine nasal spray FDA approved?
Yes. SPRAVATO® is FDA-approved for treatment-resistant depression and certain cases of MDD.
Can esketamine be used at home?
No. Esketamine must be administered in a certified healthcare setting under the REMS program.
Is esketamine the same as ketamine?
Esketamine is a derivative of ketamine but is specifically formulated and FDA-approved for nasal administration in depression treatment.
How long does esketamine treatment last?
Treatment duration varies by patient response and provider guidance.
Is esketamine legal in the US?
Yes, when prescribed and administered according to FDA and DEA regulations.
Summary
Esketamine Nasal Spray (SPRAVATO®) is a FDA-approved, prescription-only treatment for adults with treatment-resistant depression, administered under strict medical supervision through a REMS program. With a novel mechanism of action and rapid onset, esketamine represents a significant advancement in depression care when used responsibly within certified US healthcare settings.
YOU CAN ORDER KETAMINE POWDER HERE
OR ORDER XANAX 1MG HERE

Reviews
There are no reviews yet.